Compliance Dashboards for Pharma: What to Track
Why Compliance Visibility Is Non-Negotiable
In pharma, compliance isn’t just about staying legal—it’s about protecting reputations, avoiding fines, and keeping access channels open.
Compliance dashboards give visibility into key risk areas: promotional activity, field rep engagement, sample tracking, and third-party interactions. Done right, they surface what legal, medical, and compliance teams care about most.
Key Metrics to Include in a Compliance Dashboard
Field rep activity vs. approved content Cross-check rep actions (calls, emails, meetings) against approved materials to detect off-label risks or non-compliant messaging.
Sample distribution and reconciliation Track how samples are requested, shipped, and acknowledged—especially in high-risk territories or specialties.
Speaker program spend and attendance Monitor budget, HCP participation, and content delivery to flag anomalies or overuse of high-cost speakers.
External engagements and transfer of value Ensure transparency in payments to HCPs, consultants, and institutions. Dashboards help validate data before Sunshine reporting.
Data Sources That Feed Compliance Dashboards
The most effective compliance dashboards pull from:
- CRM activity logs (Veeva, Salesforce)
- Approved content repositories (e.g., DAM systems)
- Sample management platforms
- Event platforms and TOV reporting tools
By connecting these systems, teams can validate activity in near real time, not months after the fact.
Use Cases Across the Compliance Lifecycle
Monitoring field execution Compliance can proactively check for risks by region, brand, or rep type—without having to audit everything manually.
Supporting audits and inspections Dashboards provide one-click access to clean, timestamped logs and reconciliation records when regulators ask.
Partner oversight Helps manage third-party vendors involved in speaker programs, logistics, or sampling by showing exactly where risk sits.
How We Build It at Greenwolf
We tailor compliance dashboards to your risk profile, internal policies, and data landscape. Most clients ask for:
- Risk flags by territory or brand
- Audit-ready reporting packages
- Time-stamped content usage tracking
We work closely with compliance and IT to ensure the dashboard is both technically sound and regulator-ready.
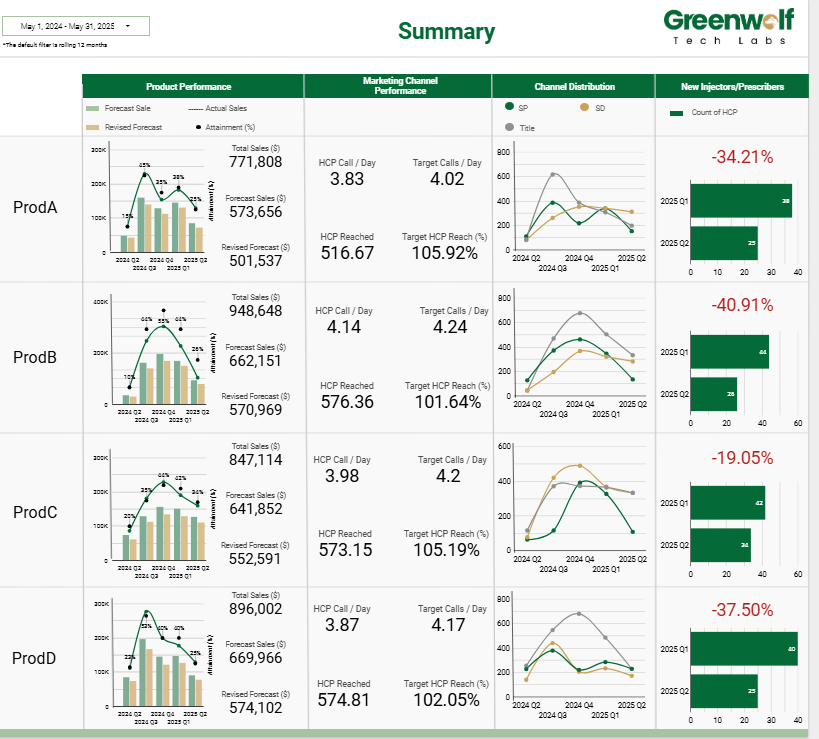
Pharma Sales Ops Dashboard
Frequently Asked Questions
What is a pharma compliance dashboard?
It’s a tool that helps compliance teams track key risk indicators across field activity, sampling, and HCP engagements.
Who uses these dashboards?
Typically used by compliance, medical affairs, legal, and sometimes brand leadership or sales ops.
Can dashboards reduce audit risk?
Yes. By surfacing red flags early, teams can act before small issues become audit findings.




